Understanding How USP 797 Affects Allergenic Compounding
USP 797 informs medical professionals preparing compounds of best practices. Besides guidance on how to set up a sterile environment and train staff, USP 797 details documentation and reporting requirements, which are essential for ensuring the quality and safety of the compounded antigens in your allergy department. Many Fuel Medical members use Allergy EDGE™ to track patients’ immunotherapy progress and manage antigen inventory, but it can also be used to document and report your compounding activities per USP 797. This Ask Fuel First section answers the following question: How can Allergy EDGE assist with USP 797 compliance?
What Is Allergy EDGE?
Allergy EDGE is a Fuel Medical cloud-based software that helps ENT and allergy practices across the nation manage their workflow, optimize the patient experience and accelerate revenue growth. Allergy EDGE also aids in compliance with USP 797, which went into effect on November 1, 2023, with features ranging from alerting staff to beyond-use dates (BUDs) to recording prescription sets.
Beyond-Use Dates
Section 21.5 of USP 797 states that prescription-set BUDs are no later than the earliest expiration date of any allergenic extract or diluent, without exceeding one year from the mixing or dilution date. Fuel Medical refers to BUDs as expiration dates. We know that without a system of tracking the hundreds of treatment vials’ expiration dates prepared each year, practices juggling these time-sensitive solutions with care may inadvertently give patients expired vials, thus failing to meet USP 797 requirements.
Allergy technicians use Allergy EDGE to set expiration dates for every vial that they mix or dilute (see Image 1. Allergy EDGE Default Expiration Date).
Image 1. Allergy EDGE Default Expiration Date
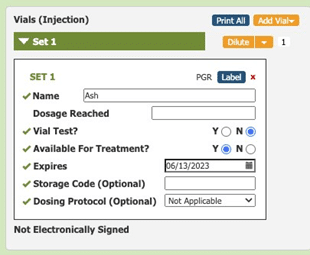
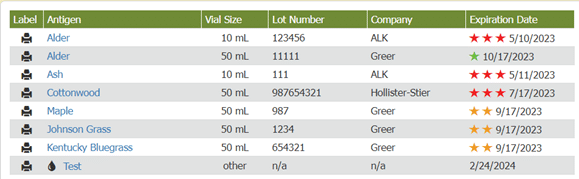
Visual cues immediately alert staff to expiration dates, helping them be more aware of vials’ shelf life and preventing waste. As long as the practice only uses vials before their expiration dates, they are in compliance with USP 797. When accessing inventory through Allergy EDGE, allergy technicians see a series of colored stars (see Image 2. Allergy EDGE Antigen Expiration Dates) indicating the following:
| One green star means an antigen won’t expire for at least 60 days. | |
| Two orange stars mean an antigen will expire within 30 days. | |
| Three red stars mean an antigen is past expiration—requiring action. |
Image 2. Allergy EDGE Antigen Expiration Dates
Labeling Patient Vials
Every vial used in an allergy department must be labeled correctly. This is important to ensure each patient gets an antigen prepared especially for them. According to 21.6 of USP 797, vials “must display the following prominently and understandably:
- Patient name
- Type and fractional dilution for each vial, with a corresponding vial number
- BUD [or expiration date]
- Storage conditions” (USP, 2022)
Allergy EDGE has been creating antigen labels for the last 12 years, well before USP 797’s mandate. Below is a sample label (see Image 3. Allergy EDGE Sample Label) generated by our software:
Image 3. Allergy EDGE Sample Label

You’ll notice that this label has the patient’s name, date of birth, set number, expiration date (or BUD) and the person who documented this label (e.g., Allan). The barcode on the left side serves as the vial number, as each one is unique. Since most practices’ workflow includes mixing off of the endpoint of an antigen’s dilution, the fractional dilution isn’t explicitly stated on the label but can be added to the name of the vial. However, all of the dilution information and history of past dilutions of an antigen in a patient’s vial are readily available. Please ask our Allergy EDGE team for more details.
Documenting Extract Prescription Sets
Besides guidance on labeling vials correctly, USP 797 discusses documentation of prescription sets. Practices must have written or electronic documentation to describe their compounding process, staff training records showing competency assessments, qualifications and corrective actions (if necessary) and temperature logs. This information was discussed in the second article, “Personnel and Facility Qualifications for USP 797,” of this Ask Fuel First section. We suggest that you keep a physical copy of these facility and personnel requirements as well as an electronic version.
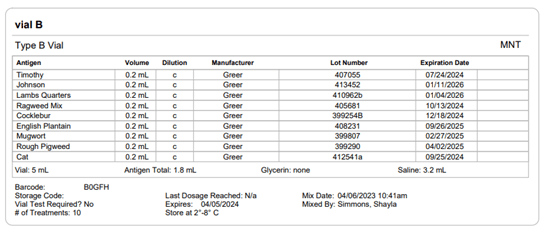
Section 21.8 of USP 797 also states that practices must keep “compounding records for individual allergenic extract prescription sets, information related to complaints and adverse events including corrective actions [and] investigations and corrective actions” (USP, 2022). Allergy EDGE records every patient’s prescription set and the outcomes aligning with USP 797 requirements (as noted in box 11 of USP 21.8, which outlines what should be included in the compounding records). All of this information is included in PDFs and views within Allergy EDGE (see Images 4–6).
Image 4. Allergy EDGE Vial Request Screenshot
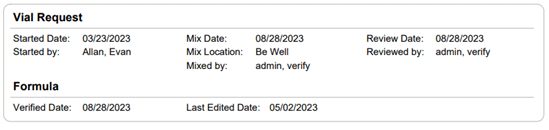
Image 5. Allergy EDGE Vial A Screenshot
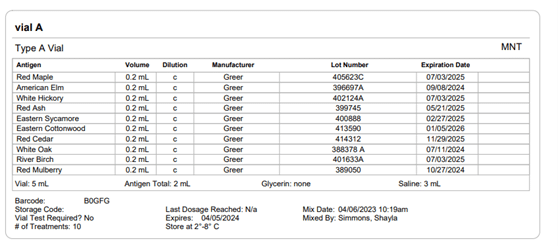
Image 6. Allergy EDGE Vial B Screenshot
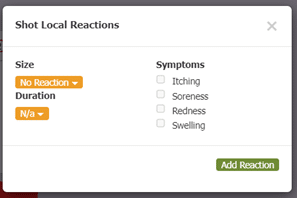
Another part of box 11 in section 21.8 includes documenting the results of QC (quality control) procedures. Allergy EDGE also does this through the mixing process (see Image 7. Allergy EDGE QC Procedures). Adverse reactions are recorded for each vial per visit (see Image 8. Allergy EDGE Shot Local Reactions), thus meeting USP 797 requirements.
Image 7. Allergy EDGE QC Procedures
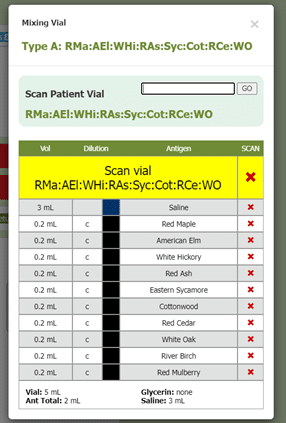
Image 8. Allergy EDGE Shot Local Reactions
Even though we’ve described quite a few of USP 797’s requirements—focusing on the needs of Fuel Medical’s members—these are simply some of our interpretations. It’s essential that you read through the original text, as USP 797 is mandated by law. As we meet with professionals in the field of immunology and discuss the implications of USP 797, we’re confident that adopting Allergy EDGE is the first step in compliance. Contact Fuel Medical’s Allergy EDGE team at Allergy@FuelMedical.com for more information.
References
United States Pharmacopeia (2022). General Chapter, (797) Pharmaceutical Compounding—Sterile Preparations. USP-NF. Rockville, MD: United States Pharmacopeia. https://doi.org/10.31003/USPNF_M99925_06_01.
